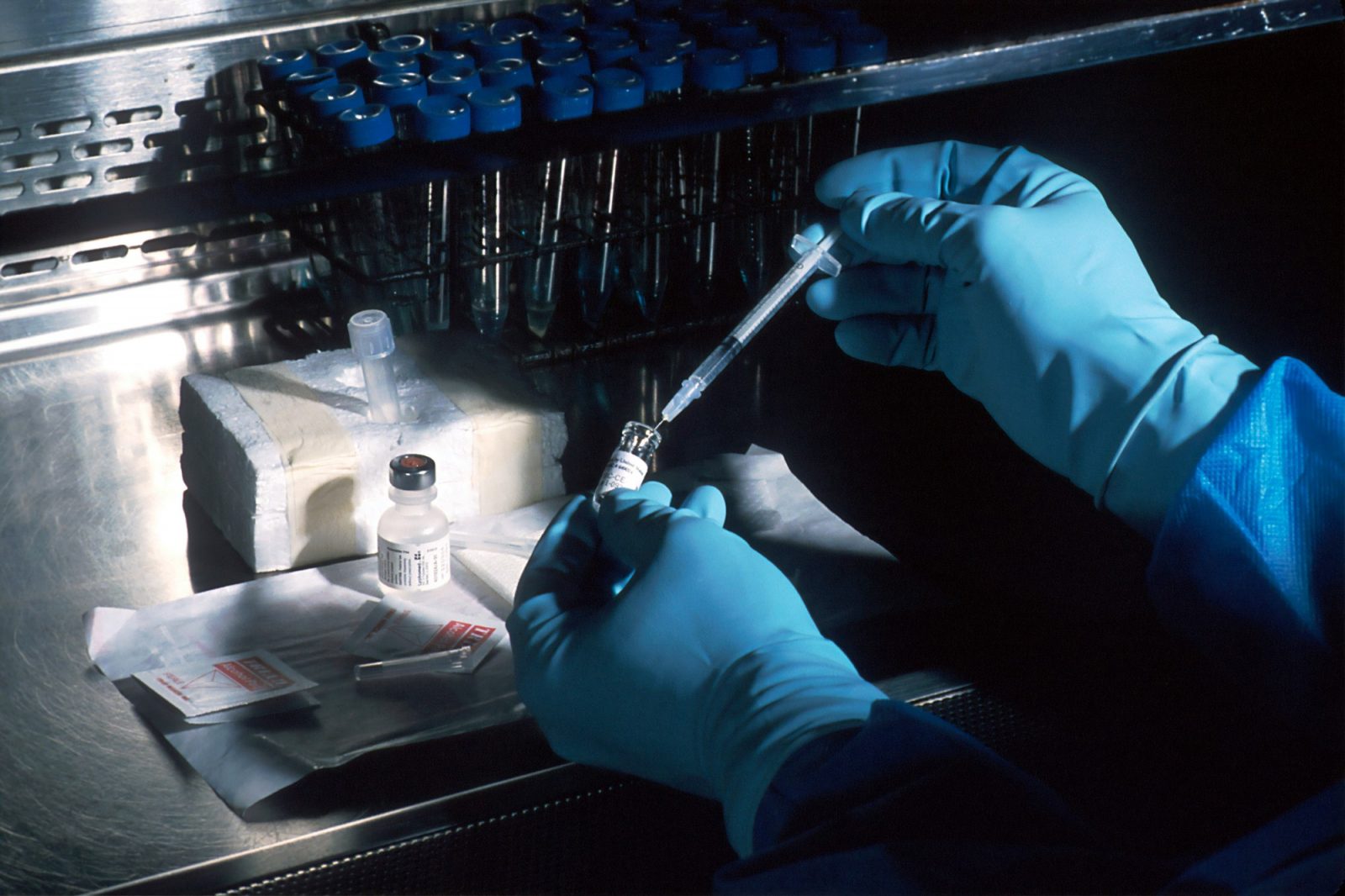
It’s one of approximately 12 experimental vaccines currently or soon to begin human testing worldwide.
Australia has launched its first human trials for potential COVID-19 vaccine NVX-CoV2373 in Melbourne. The vaccine, developed by US biotech company Novavax, is being tested by clinical research organisation Nucleus Network.
The trials will involve 131 healthy volunteers between 18 and 59 years old, with testing currently taking place in Melbourne and soon to begin in Brisbane.
In a virtual press conference, Novavax chief scientific officer Gregory Glenn said that the trial will move very quickly, with phase one results expected by the end of July and phase two by the end of the year.
“Everything is very accelerated, vaccines can take eight to ten years to develop,” he said. “Obviously we need a vaccine so we’re trying to compress everything.”
Glenn also said the company has been working on a vaccine since the outbreak began in China at the beginning of the year, with NVX-CoV2373 chosen from a pool of 30 potential vaccines. He also stressed that making the vaccine globally available is a priority, with phase two trials set to take place across various countries, involving thousands of volunteers.
NVX-CoV2373 is one of about a dozen experimental vaccines currently or soon to commence human testing worldwide, according to SBS, so it’s possible we might see a coronavirus vaccine in the near future.
Microbiologist Paul Griffin told the ABC that volunteers for human trials of NVX-CoV2373 are still needed and encouraged those willing to take part to sign up.
Find out more here.
Never miss a story. Sign up to Beat’s newsletter and you’ll be served fresh music, arts, food and culture stories three times a week.